Question
Preview
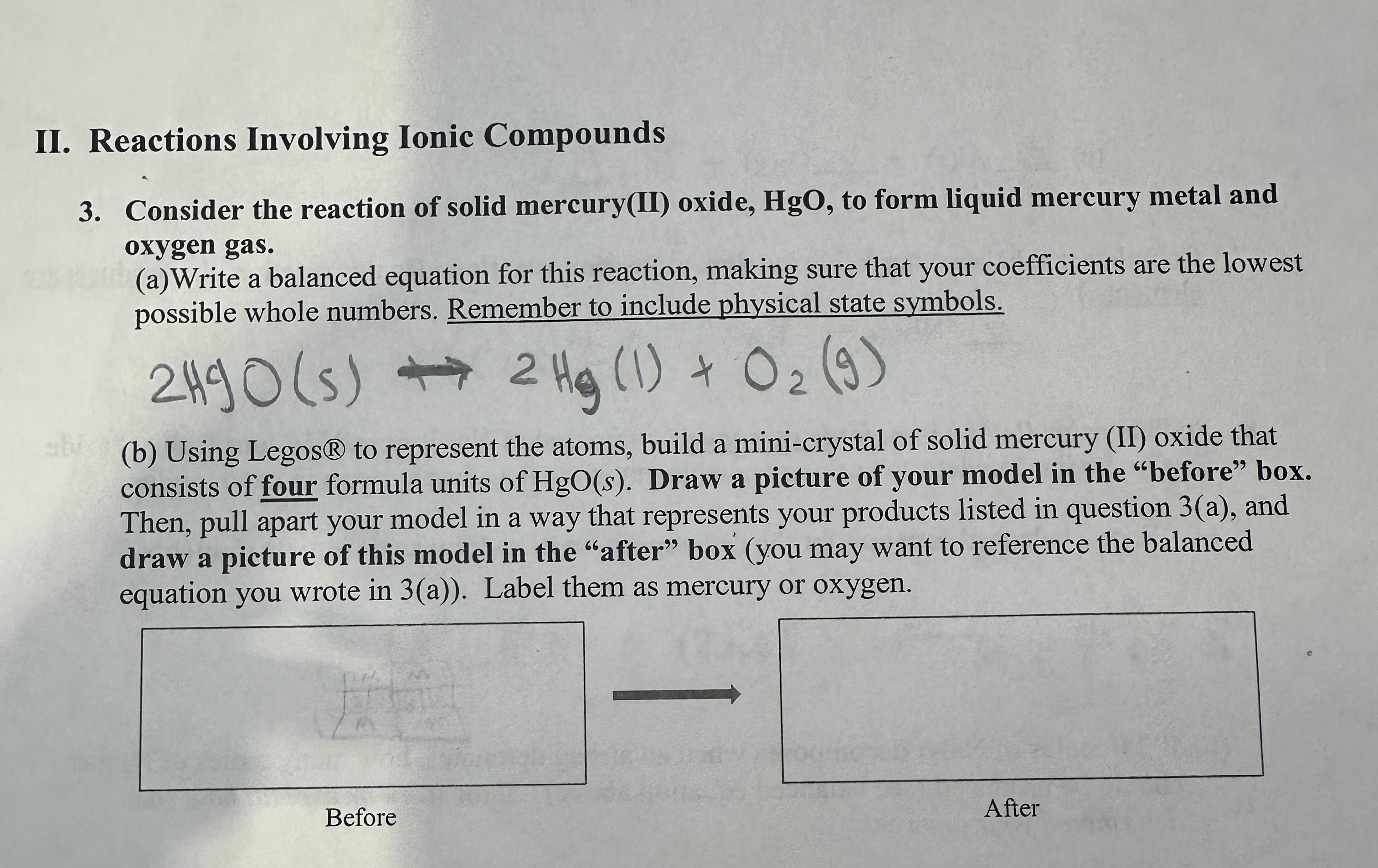
II. Reactions Involving Ionic Compounds
3. Consider the reaction of solid mercury(ll) oxide, HgO, to form liquid mercury metal and
oxygen gas.
(a)Write a balanced equation for this reaction, making sure that your coefficients are the lowest
possible whole numbers. Remember to include physical state symbols.
( 02
(b) Using Legos@ to represent the atoms, build a mini-crystal of solid mercury (II) oxide that
consists of four formula units ofHgO(s). Draw a picture of your model in the "before" box.
Then, pull apart your model in a way that represents your products listed in question 3(a), and
draw a picture of this model in the "after" box (you may want to reference the balanced
equation you wrote in 3(a)). Label them as mercury or oxygen.
Before
After
Answer
Chrome Extension
1. Search answers from our 90+ million questions database.
2. Get instantly AI Solutions powered by most advanced models like GPT-4, Bard, Math GPT, etc.
3. Enjoy one-stop access to millions of textbook solutions.
4. Chat with 50+ AI study mates to get personalized course studies.
5. Ask your questions simply with texts or screenshots everywhere.
Recommend videos
Why AI Homework